Clinical and industrial studies
Clinical and industrial studies
Clinical and industrial studies
Clinical research includes all scientific studies conducted on humans as part of the development of a medical device. Its aim is to develop and assess the service provided by new medical devices in order to ensure better patient care, while improving their health and quality of life.
With more than 30 years of medico-technical expertise and integrated into a network of clinical establishments, INI-CERAH has been offering support for the conduct of clinical studies for several years, particularly on assistive products. From the definition of the protocol to the complete management of the study, CERAH will support you in your clinical study project according to your needs.
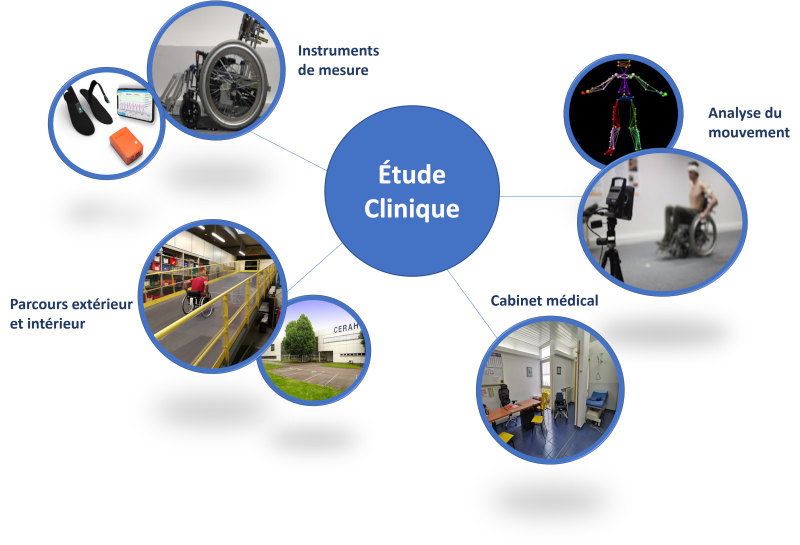
Our competences :
- Data acquisition (including biomechanics)
- Processing and analysis
- Statistics
- Creation of a protocol
- Patient recruitment
- Coordination and clinical follow-up
- Establishment of CPP, application for authorisation ANSM
- Drafting of the registration file LPPr
With its dedicated spaces and equipment (movement capture, effort measurement, courses, etc.), our centre allows a wide range of clinical investigations to be carried out. For more information, contact us.

